Difference Between Capacitor and Battery
A battery is an electronic device that changes chemical energy into electrical energy to supply a steady flow of power. In contrast, a capacitor is a component that stores electrostatic energy in an electric field and releases it quickly when needed. While both devices store and deliver energy, they do so through very different principles. For example, a battery works like a fuel tank that provides energy slowly over time, while a capacitor acts more like a water balloon that can discharge energy almost instantly.
Both the capacitor and the battery are used in electrical and electronic circuits for energy storage and charging, but their behavior under conditions like temperature, voltage, and life cycle varies greatly. The following table highlights the main differences between a capacitor and a battery based on these key performance factors.
Watch This Video to Understand the Real Difference Between Capacitor and Battery!
What is a Capacitor?
A capacitor is an electronic component that stores electrical energy in an electric field. It is made of two conductive plates separated by a thin insulating material called a dielectric. When a voltage is applied across these plates, positive charges gather on one plate and an equal amount of negative charges collect on the other. This separation of charges creates an electric field between the plates, allowing the capacitor to store energy. The ability of a capacitor to hold this charge is known as its capacitance.
You can think of a capacitor as a small energy container that fills up quickly and empties just as fast when needed, much like how a camera flash charges and releases energy in an instant. The amount of charge a capacitor can store depends on its size, plate area, distance between plates, and the type of dielectric used. Modern capacitors come in many forms and materials, such as ceramic, electrolytic, and film types, each designed for specific applications in electrical and electronic circuits.
The capacitance of a capacitor is given by the formula:
C = Q / V
where C is the capacitance in farads (F), Q is the electric charge in coulombs (C), and V is the voltage across the plates in volts (V).
What is a Battery?
A battery is a device made up of one or more electrochemical cells that convert chemical energy into electrical energy. In simple terms, it works like a small chemical power plant where controlled reactions produce electricity. Each cell contains two electrodes—a positive and a negative terminal—between which redox reactions (oxidation and reduction) take place. These reactions cause electrons to flow through an external circuit, providing electrical power to devices such as flashlights, remote controls, or mobile phones.
A battery functions as a galvanic cell, meaning it generates electricity directly from chemical reactions. The electrodes inside the battery are the key sources of this energy, as they contain the reactive materials that release or absorb electrons during operation. Over time, these materials get used up, which limits the battery’s lifespan or requires recharging, depending on the type.
There are two main types of batteries:
-
Primary Cell / Primary Battery – These are non-rechargeable batteries designed for single use. Once their chemical energy is exhausted, they must be replaced. Common examples include alkaline and zinc-carbon batteries used in toys or remotes.
-
Secondary Cell / Secondary Battery – These are rechargeable batteries that can be used multiple times by restoring the chemical energy through charging. Examples include lithium-ion, lead-acid, and nickel-cadmium batteries.
Batteries are also classified according to their applications, such as household batteries, industrial batteries, automotive batteries, and portable electronic batteries, each designed to meet specific power and durability needs.
Main Differences Between a Battery and a Capacitor
The table below highlights the key differences between a battery and a capacitor, explaining how each device stores, releases, and manages energy in electrical and electronic circuits.
| Characteristics | Battery | Capacitor |
|---|---|---|
| Symbol | 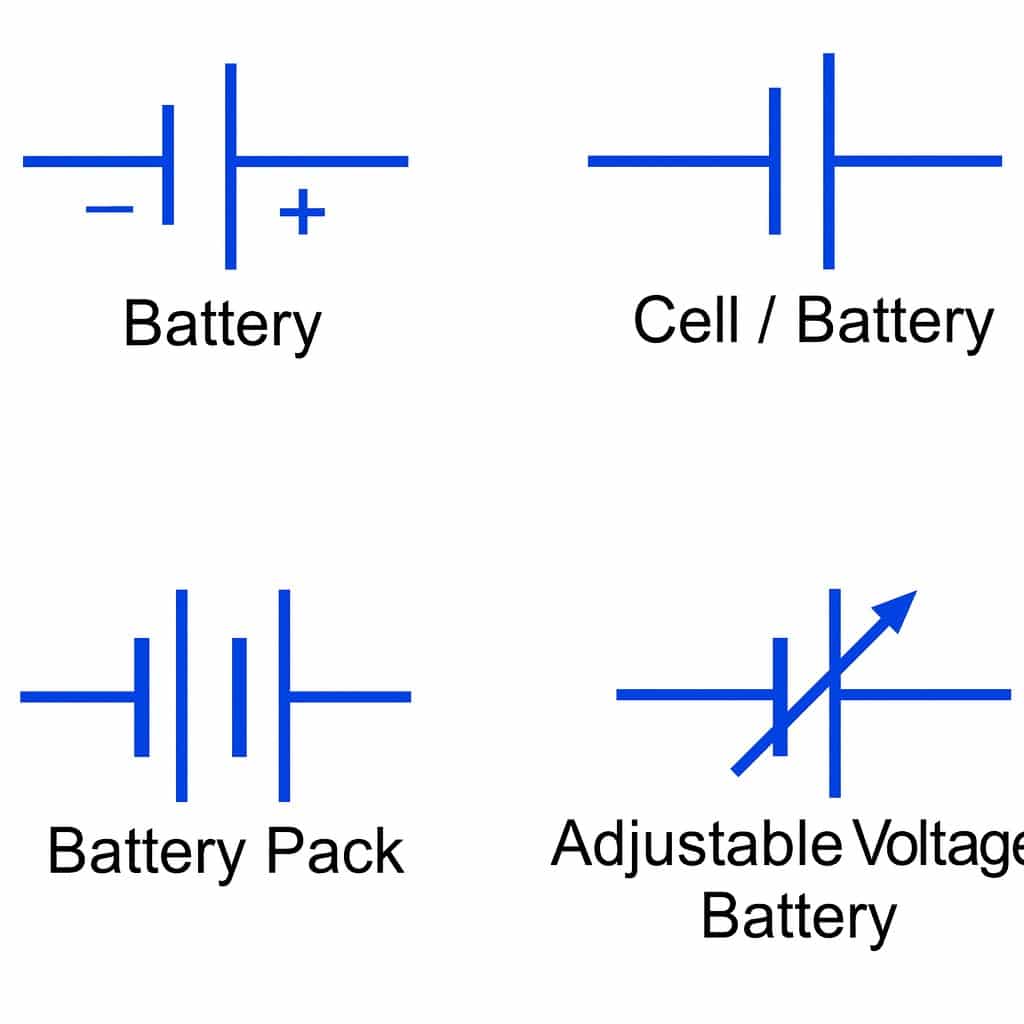
|
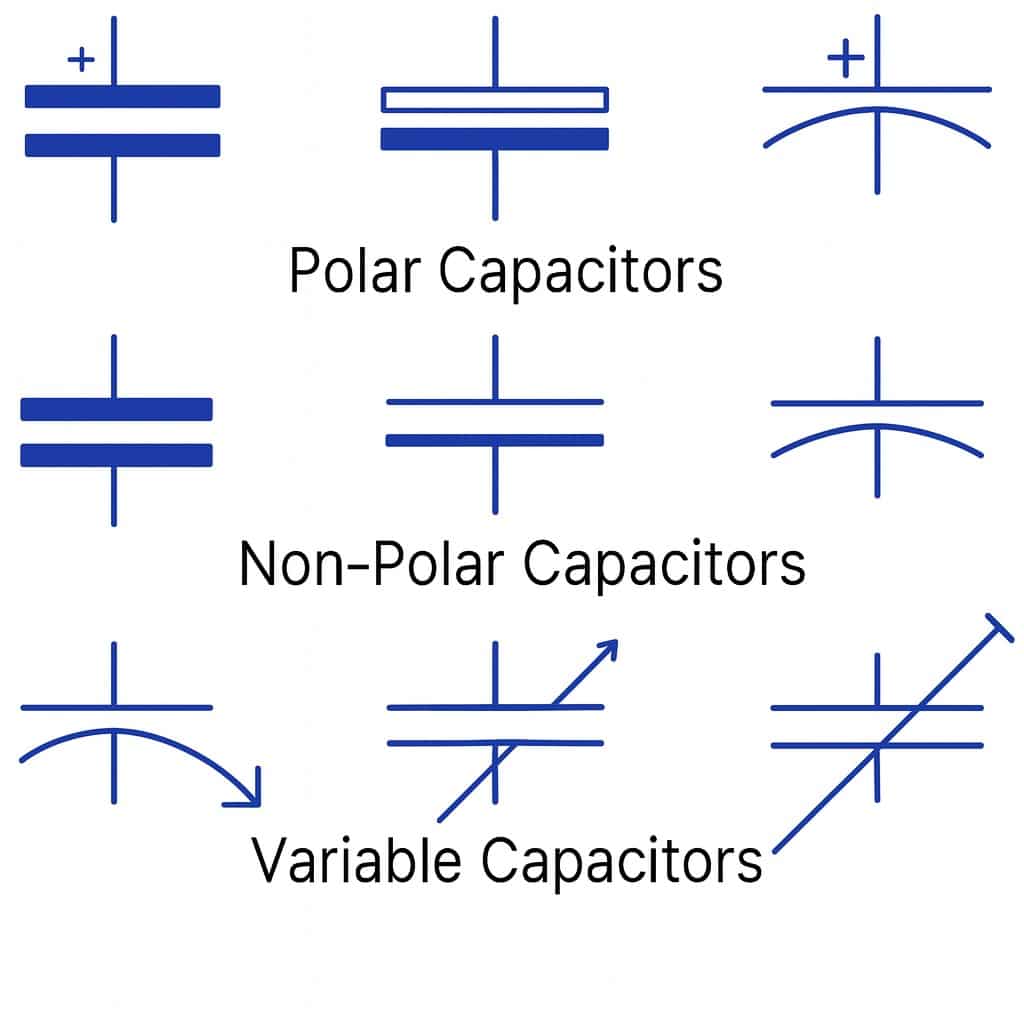
|
| Definition | A battery stores potential energy in the form of chemical energy, which is later converted into electrical energy through chemical reactions. | A capacitor stores potential energy in an electric field (electrostatic field) and releases it to the circuit as electrical energy. |
| Construction | A battery has three main parts: the cathode (positive terminal), the anode (negative terminal), and the electrolyte (a medium that allows ions to flow). | A capacitor is a two-terminal device made of metal plates separated by a dielectric material (an insulator). |
| Function | The battery supplies electrical energy to the circuit by generating and moving electrons through a chemical process. | The capacitor draws, stores, and releases energy. It does not generate electrons—it only stores existing charge. |
| Working Principle | A battery operates on oxidation–reduction (redox) reactions, which produce electrical energy. | When voltage is applied across its terminals, a capacitor stores energy in the electric field formed between its plates. |
| Operation | A battery generates electrons through chemical reactions. | A capacitor stores electrons on its plates. |
| Types | Alkaline, Lithium, Silver Oxide, Zinc-Air, Zinc-Carbon, Lead-Acid, Li-ion, Ni-MH, and Ni-Cd. | Electrolytic, Electrostatic, Electrochemical, Supercapacitor, Hybrid Supercapacitor, Ceramic, Film, Tantalum, and Integrated Capacitors. |
| Type of Device | Active component, since it produces energy. | Passive component, since it only stores energy. |
| AC & DC Usage | Used mainly to provide DC supply. | Blocks DC but passes AC signals. |
| Voltage Behavior | Provides a constant voltage during discharge. | Voltage drops rapidly during discharge. |
| Potential Difference (P.d) | Constant during operation. | Increases exponentially while charging. |
| Charging & Discharging Time | Slow, usually between 10–60+ minutes. | Very fast, typically 1–10 seconds. |
| Charging Temperature | Works best between 0–45°C (32–113°F). | Operates within –40 to 65°C (–40–149°F). |
| Life Cycle | Around 500+ hours. | Between 1M–3M hours (much longer). |
| Service Life | About 5–10 years. | About 10–15 years. |
| Voltage per Cell | 3.6–3.7 V per cell. | 2.3–2.75 V per cell. |
| Specific Power Rating | Around 1k–3k W/kg. | Around 1M W/kg, meaning it can deliver bursts of energy much faster. |
| Polarity | Polarity reverses during charging and discharging. | Polarity must remain the same during both charging and discharging. |
| Size | For the same storage capacity, a battery is usually smaller. | A capacitor is larger for the same energy capacity. |
| Cost | Higher cost compared to capacitors. | Lower cost for similar applications. |
| Advantages | • High storage capability • Good power density • Better leakage control than capacitors • Constant voltage output • Long life cycle |
• Short charging time • Handles high load currents • Works well under temperature variations • Long operational lifespan |
| Disadvantages | • Limited life cycle • Long charging time • Sensitive to temperature • Voltage and current limits |
• Low specific energy • High self-discharge rate • Expensive per watt compared to batteries • Linear voltage discharge during operation |
| Applications | • Power electronics and household devices • Energy storage and medical equipment • IoT, AI, and military systems • Automotive and backup power |
• Power smoothing and power factor correction • High-pass and low-pass filters • Signal coupling and decoupling • Motor starters, snubbers, and oscillators |
In summary, a battery is best for long-term, steady energy supply, while a capacitor is ideal for quick energy bursts. In many electronic systems, both are used together—just like how a water tank (battery) provides a steady flow and a spring (capacitor) gives short bursts of energy when needed.
Frequently Asked Questions – FAQs
Q1. What happens when a capacitor is connected to a battery?
When a capacitor is connected to a battery, electric charge begins to build up on both plates of the capacitor. Electrons move from one plate to the other, creating a temporary current flow in the circuit. As the capacitor becomes fully charged, this current gradually decreases until it eventually reaches zero. At that point, the capacitor holds a steady voltage equal to the battery’s voltage, similar to how a water tank fills up and then stops flowing once it’s full.
Q2. Where is energy stored in the capacitor?
The energy in a capacitor is stored in the electric field that forms between its two plates. This field exists in the space between the plates, where the dielectric material is placed. The amount of energy stored depends on the electric charge accumulated on the plates and the potential difference (voltage) across them.
Q3. How is the storage battery rated in capacity?
The capacity of a storage battery represents how much charge it can deliver over time. It is defined as the product of the current (in amperes) drawn from the battery and the time (in hours) for which the current flows. The unit used for measuring battery capacity is the ampere-hour (Ah). For example, a 10 Ah battery can supply 1 ampere for 10 hours or 2 amperes for 5 hours.
Q4. What is the number of positive plates in a battery cell?
In a battery cell, the number of positive plates is always one less than the number of negative plates. This arrangement ensures that every positive plate is positioned between two negative plates, allowing efficient chemical reactions and better current distribution inside the cell.
Q5. What is the emf of the dry cell?
The electromotive force (emf) of a standard dry cell is 1.5 volts (V). This is the typical voltage produced by common batteries such as alkaline or zinc-carbon cells used in flashlights, remotes, and clocks.